Proper sample preparation is essential to getting highly quality XRD data. If you do not put in the effort to properly prepare your sample you can introduce errors that make phase identification difficult to impossible and estimates of abundances and crystallinity erroneous. Ideally, you need to achieve three conditions in order to have good data:
- Total randomness of crystallite orientations
- Sufficient number of crystallites to get a representative intensity distribution for the sample
- Sufficient diffraction intensity to meet satisfy counting statistics
The best way to make sure these conditions are met is to finely powder your sample.
Why We Powder Samples
Errors Caused By Materials and Improper Preparation
Grinding Samples
Why We Powder Samples
Particle size is inversely related to both the degree of randomness of the crystallites and the measured intensity. The figure below illustrates this relationship for a single phase sample prepared properly, then poorly.
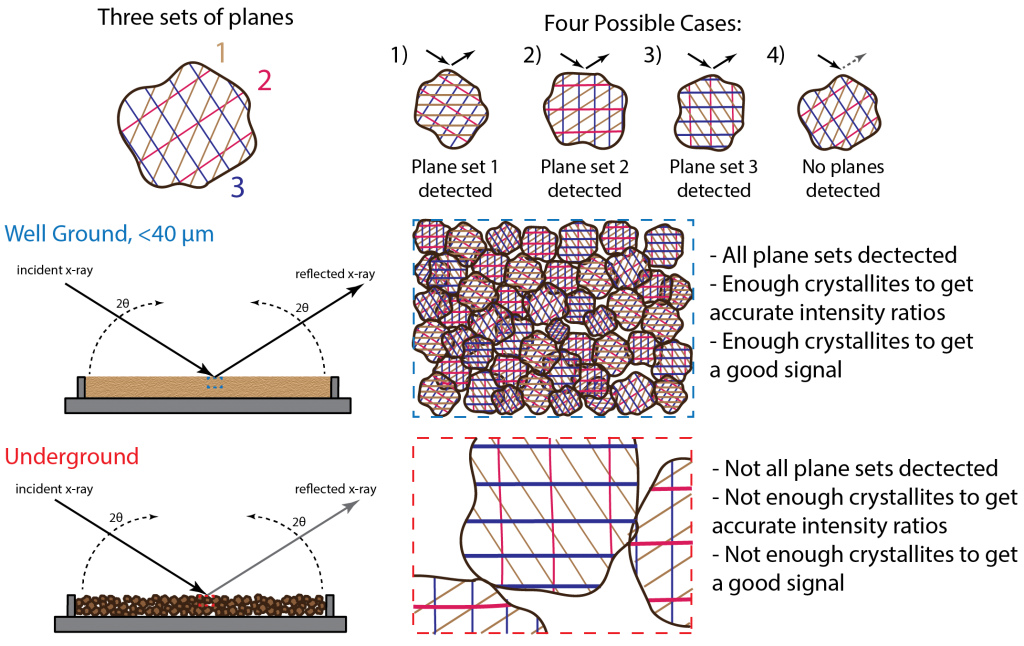
The well ground sample has a particles size of at most 44 microns. It would resemble a flour such that if you were to rub the sample between your fingers you would not be able to feel the individual grains. The underground sample you would be able to distinguish individual grain not only by feel but sight as well. The resulting diffraction patterns of the two samples are shown below.
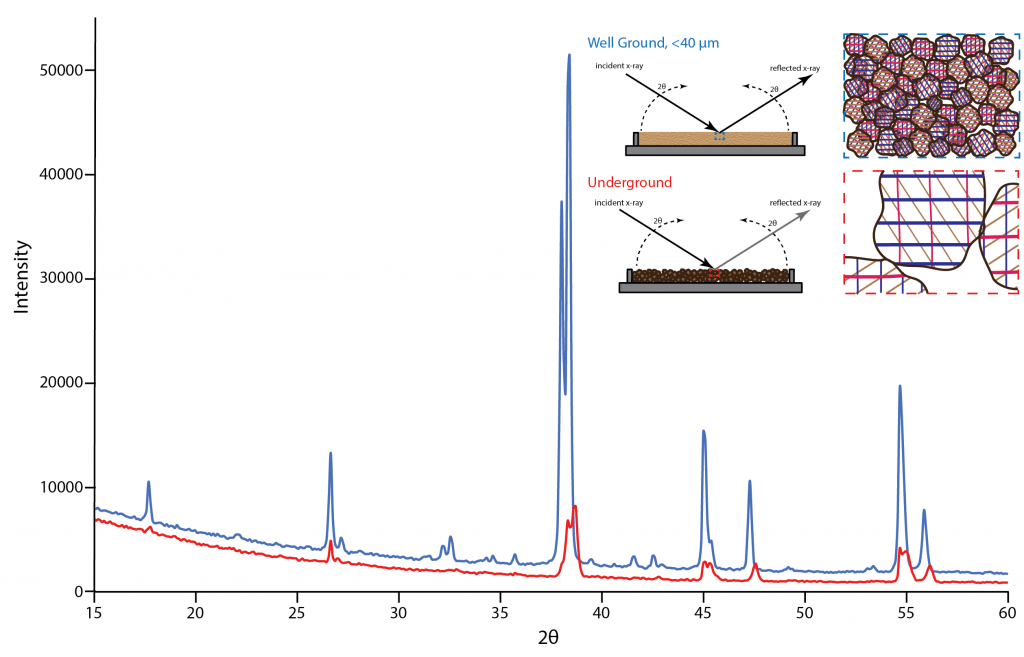
The difference between the patterns is immediately obvious. The well ground sample's diffraction pattern has a high signal to background ratio and the peak ratios match those seen in other collected diffraction patterns of this material. The underground sample's pattern has a low signal to background ratio; to the point that some of the smaller peaks detected in the well ground sample pattern disappear into the background noise. Also, the ratios of the peaks are off indicating the crystallites were not sufficiently random in their orientations.
This demonstrates just how important it is that you make sure that your sample is completely ground to a flour. If you can see or feel individual grains of your sample you need to keep on grinding. The effort you put in does have a huge impact on the diffraction pattern you will receive and heavily influence your ability to subsequently identify, characterize, and interpret your sample.
Errors Caused by Materials and Improper Preparation
Some samples are easier to prepare than others. Depending on the hardness, habit, reactivity, etc. of the phase(s) in the sample different preparation techniques are required. It is important that you are aware of these obstacles to ensure you apply the correct techniques and care in preparing your sample or are aware what the errors look like when interpreting a diffraction pattern. In this section we will cover some samples that require extra care and the various consequences of improper sample preparation.
Preferred Orientation
Preferred orientation occurs when a samples crystallites do not randomly orientate because of its texture other physical properties. Samples that are fibrous, bladed, tabular, or platy will want to orientate themselves in a preferred direction due to their shape, causing some lattice planes to be detected more than others and therefore causing the intensity ratios to be skewed (See figure below).
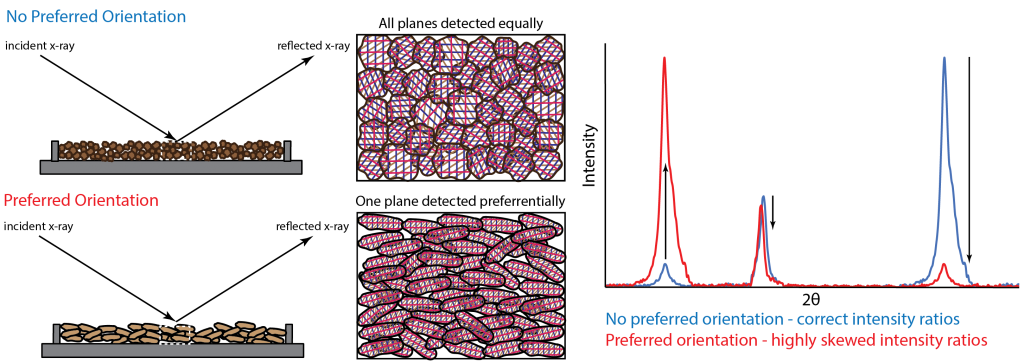
This problem can be mitigated by proper grinding and mounting of a sample to encourage random orientation. However preferred orientation cannot always be avoided. In this case, it is helpful to know what phases are prone to this problem and account for this in any subsequent data interpretation.
Preferred orientation also is a prominent problem in samples that have not been ground (i.e. steels, wafers, polymers). It is important to be aware that the position that the sample is mounted in changes the diffraction pattern measured dramatically. Different orientations cause intensity ratios to widely vary or for some peaks not to be detected at all. The figure below shows an example of this. The initial orientation of the sample when analyzed led to many peaks not being detected. Just rotating the analyzed face by 90 degrees fixed this problem.
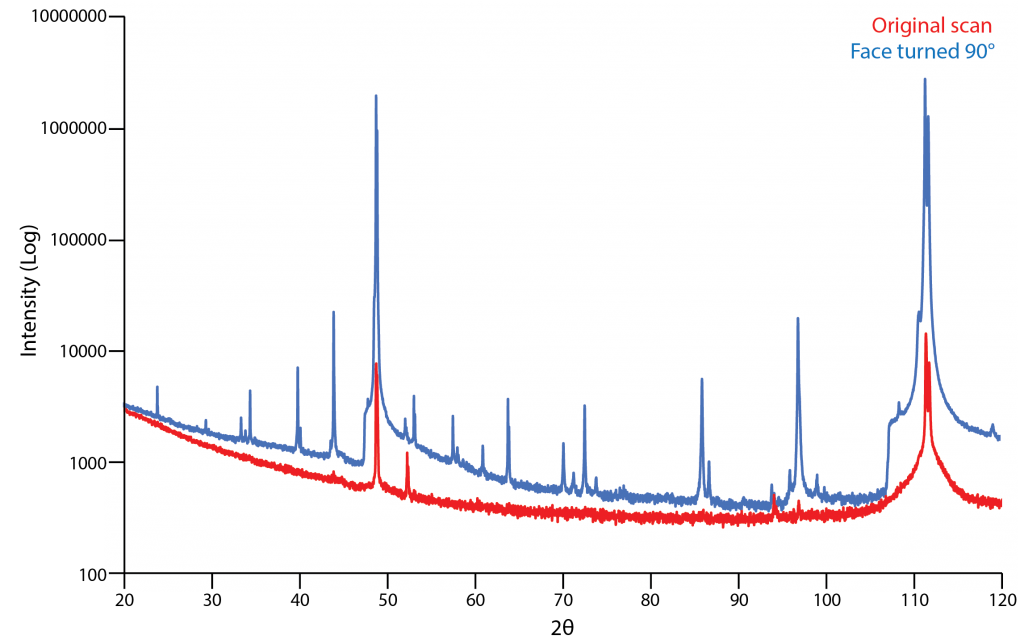
Errors Caused by Improper Preparation
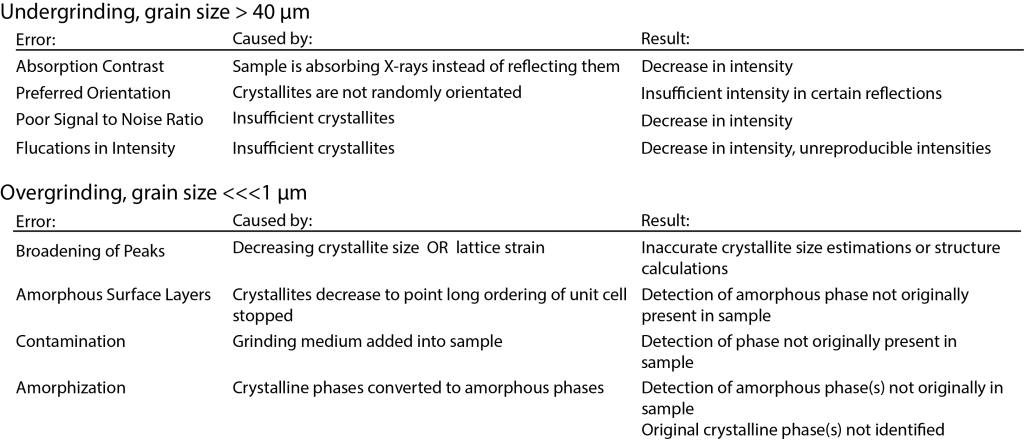
Grinding Samples
Hand Grinding:
Usually powdered XRD samples are prepared by hand grinding using a mortar and pestle. The mortar and pestle can be made out of a variety of materials such as agate, corundum, or mullite. The type you use will depend on the hardness of your sample compared to the material of the mortar and pestle and whether contamination introduced by the grinding medium will effect subsequent interpretations of your data. Typically samples are ground under a liquid medium like ethanol or methanol to minimize sample loss during grinding and to mitigate structural damage to the phases in your sample that can be caused by grinding. It can however be tedious and physically demanding to hand grind samples, especially if your sample is very hard or has other physical properties making it difficult to grind.
Mechanical Grinding:
Though it is possible to hand grind a sample to 1 μm, as mentioned above, it is tedious and difficult. Mechanical grinders are an excellent way to grind your sample to grain sizes approaching 1 μm for quantitative XRD work. This can be done with shatter boxes or ball mills, but both of these produce a large particle size distributions. McCrone Mills are the best way to produce both small grain sizes and narrow size distributions. They consist of a Teflon cup holding pellets made of either agate, corundum, or tungsten carbide which is then loaded into a holder that shakes the pellets at a high frequency for a set amount of time. Again, a liquid medium like ethanol is used to reduce lattice stain during milling. The draw backs of this method is only a small amount of sample can be processed and there is always a little bit of contamination (< 1 wt%).


References: Buhrke, V. E., Jenkins, R., & Smith, D. K. (1998). A practical guide for the preparation of specimens for x-ray fluorescence and x-ray diffraction analysis. New York: Wiley-VCH.
